Overview
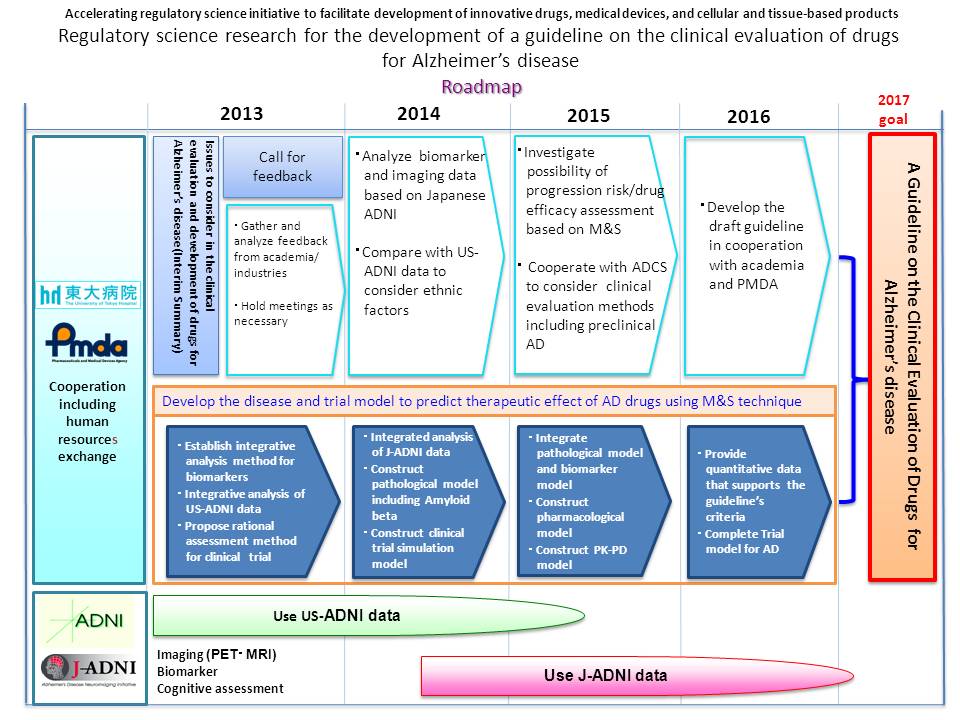
The Ministry of Health, Labour and Welfare started in 2012 the "Initiative to facilitate development of innovative drugs, medical devices, and cellular and tissue-based products". Aiming to put innovative drugs and medical devices to practical use, this initiative will develop guidelines necessary for their evaluation.
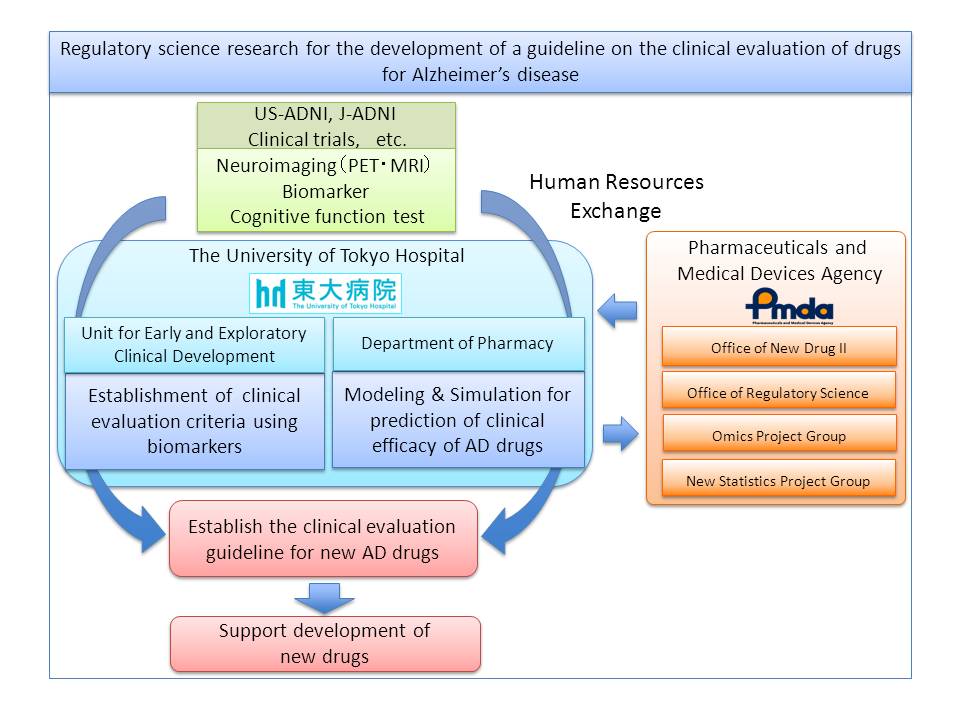
Moreover, human resource are exchanged between the PMDA (Pharmaceutical and Medical Devices Agency) and universities in order to cultivate human resource who can evaluate the safety and effectiveness of the most advanced technologies.
Promoting the development of drugs for Alzheimer's disease
The University of Tokyo Hospital is closely cooperating with the PMDA to push forward the researches below as part of the "Regulatory science research for the development of clinical evaluation criteria for drugs for Alzheimer's disease".
- Research for developing the clinical evaluation criteria of drugs for Alzheimer's disease that utilizes biomarkers (glossary).
- Research for developing a model for predicting clinical efficacy of drugs for Alzheimer's disease that utilizes modeling and simulation (glossary) technique.
The goal is to internationally propose how clinical evaluation of drugs for Alzheimer's disease should be carried out by utilizing the data of J-ADNI (Japanese Alzheimer's Disease Neuroimaging Initiative) , and to promote the development of new drugs in Japan.
------ここから後日公開部分------With the full cooperation of the PMDA, we have compiled the current approaches, points to consider and future challenges, regarding the clinical evaluation and clinical development of Alzheimer's disease modifying drugs, as the interim result of the "Regulatory science research for the development of clinical evaluation criteria for Alzheimer's disease medications. (See below reference)
Clinical trials utilizing biomarkers still face many challenges, but we aim to continue the research and to thoroughly review the amassed evidence in order to establish the final clinical evaluation guideline for drugs for Alzheimer's disease at the next step.
Reference
Issues to consider in the clinical evaluation and development of drugs for Alzheimer's disease (interim summary):
PDF format
[Glossary]
- Biomarker
- Biomarker is a characteristic that reflects the disease's diagnosis, stage of progression or therapeutic effects of drugs, and includes physiological indicators such as blood pressure and molecules including proteins and peptides, genetic information, and x-ray images. Biomarkers for Alzheimer's disease include amyloid-β and tau in the cerebrospinal fluid, PET imaging and MRI images.
- Modeling and simulation
- This refers to mathematical modeling of the relationship among pharmacokinetics, pharmacodynamics, biomarkers and clinical effectiveness. The model is used to carry out simulations of clinical trials for reviewing appropriate clinical trial design as well as decision-making related drug development.